
Chemistry, 12.03.2021 03:00 rustjallison9928
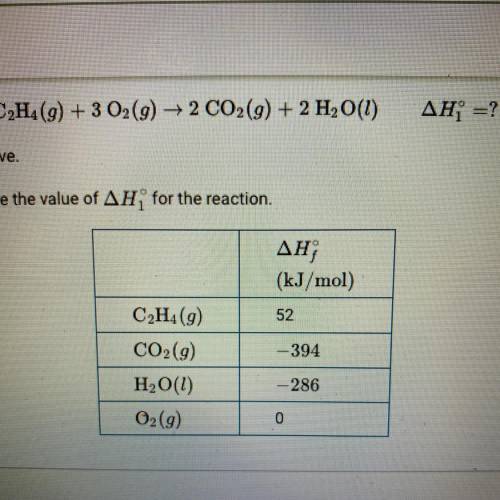
C2H4(g) + 3O2(g) —> 2CO2(g) + 2H2O(g)
change in H1 = ?
The combustion of C2H4 is represented by the equation above.
Use the enthalpies of formation in the table the calculate the value of change in H1 for the reaction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
C2H4(g) + 3O2(g) —> 2CO2(g) + 2H2O(g)
change in H1 = ?
The combustion of C2H4 is rep...
The combustion of C2H4 is rep...
Questions

Mathematics, 01.10.2019 09:30

History, 01.10.2019 09:30

Biology, 01.10.2019 09:30

Mathematics, 01.10.2019 09:30


Social Studies, 01.10.2019 09:30

Biology, 01.10.2019 09:30




Physics, 01.10.2019 09:30

History, 01.10.2019 09:30


Geography, 01.10.2019 09:30

Mathematics, 01.10.2019 09:30

Health, 01.10.2019 09:30

History, 01.10.2019 09:30

Mathematics, 01.10.2019 09:30

Mathematics, 01.10.2019 09:30



