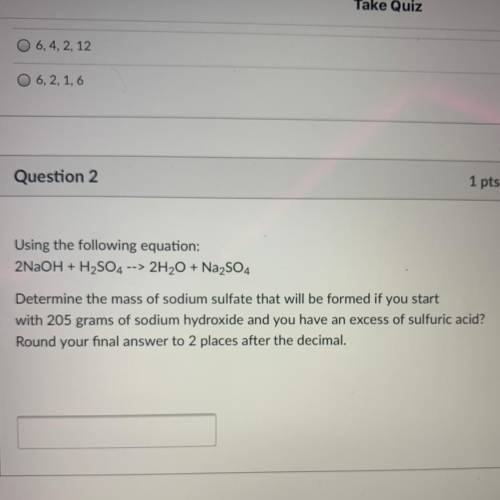
Using the following equation:
2NaOH + H2SO4 --> 2H2O + Na2SO4
Determine the mass of sodium...

Chemistry, 12.03.2021 08:20 jordan8037
Using the following equation:
2NaOH + H2SO4 --> 2H2O + Na2SO4
Determine the mass of sodium sulfate that will be formed if you start
with 205 grams of sodium hydroxide and you have an excess of sulfuric acid?
Round your final answer to 2 places after the decimal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
Questions


Mathematics, 29.08.2020 20:01

Social Studies, 29.08.2020 20:01

Computers and Technology, 29.08.2020 20:01

Mathematics, 29.08.2020 20:01

Mathematics, 29.08.2020 20:01




History, 29.08.2020 20:01







Mathematics, 29.08.2020 20:01

History, 29.08.2020 20:01

Computers and Technology, 29.08.2020 20:01



