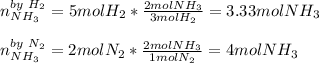If you have 5 mol H2 and 2 mol N2, what is the limiting reagent in the reaction
below?
3 H2(g...

Chemistry, 12.03.2021 08:40 gabbym39077
If you have 5 mol H2 and 2 mol N2, what is the limiting reagent in the reaction
below?
3 H2(g) + N2 (g) → 2 NH3 (g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Questions


Mathematics, 09.09.2020 22:01


Mathematics, 09.09.2020 22:01

Biology, 09.09.2020 22:01

English, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01

Physics, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01


Mathematics, 09.09.2020 22:01



Health, 09.09.2020 22:01


Mathematics, 09.09.2020 22:01