2 moles of sodium phosphate reacts with 3 moles of
calcium chloride
2Na3PO4 + 3 CaCl2 -> 6...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Questions



Biology, 21.07.2019 15:30

Mathematics, 21.07.2019 15:30



Mathematics, 21.07.2019 15:30


Mathematics, 21.07.2019 15:30

Mathematics, 21.07.2019 15:30

Mathematics, 21.07.2019 15:30


History, 21.07.2019 15:30

Mathematics, 21.07.2019 15:30

Social Studies, 21.07.2019 15:30

English, 21.07.2019 15:30

Mathematics, 21.07.2019 15:30

Mathematics, 21.07.2019 15:30

Mathematics, 21.07.2019 15:30

History, 21.07.2019 15:30


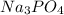 require 3 moles of
require 3 moles of 



