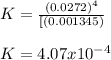Chemistry, 12.03.2021 15:20 pickelswolf3962
Nickel carbonyl decomposes to form nickel and carbon monoxide, like this:
Ni(CO)4(g) → Ni(s)+ 4CO(g)
At a certain temperature, a chemist finds that a 2.6 L reaction vessel containing a mixture of nickel carbonyl, nickel, and carbon monoxide at equilibrium has the following composition:
compound amount
Ni(CO)4 0.597g
Ni 12.7g
CO 1.98g
Required:
Calculate the value of the equilibrium constant for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Nickel carbonyl decomposes to form nickel and carbon monoxide, like this:
Ni(CO)4(g) → Ni(s)+ 4CO(g...
Questions



Mathematics, 20.04.2021 03:40

Biology, 20.04.2021 03:40


History, 20.04.2021 03:40

Biology, 20.04.2021 03:40


Mathematics, 20.04.2021 03:40

Mathematics, 20.04.2021 03:40

Mathematics, 20.04.2021 03:40

SAT, 20.04.2021 03:40

Mathematics, 20.04.2021 03:40


Mathematics, 20.04.2021 03:40

Mathematics, 20.04.2021 03:40


Arts, 20.04.2021 03:50


History, 20.04.2021 03:50

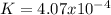
![K=\frac{[CO]^4}{[Ni(CO)_4]}](/tpl/images/1190/9822/9636a.png)
![[CO]_{EQ}=\frac{1.98g}{28.01g/mol} *\frac{1}{2.6L}=0.0272M](/tpl/images/1190/9822/0dde7.png)
![[Ni(CO)_4]_{EQ}=\frac{0.597g}{170.73g/mol} *\frac{1}{2.6L}=0.001345M](/tpl/images/1190/9822/2339a.png)