Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
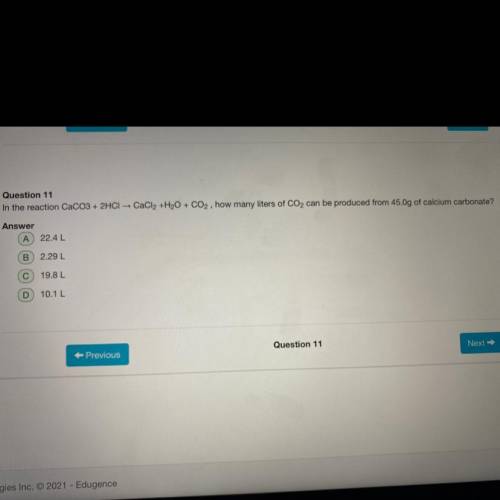
In the reaction CaCO3+2HCl CaCl 2 +H 2 O+CO 2 how many liters of CO 2 can be produced from 45.0g of...
Questions




Physics, 17.07.2020 21:01



Business, 17.07.2020 21:01







Mathematics, 17.07.2020 21:01






Mathematics, 17.07.2020 21:01