
Chemistry, 12.03.2021 21:30 destineecoleman2001
The purification of hydrogen gas by diffusion through a palladium sheet was discussed in Section 5.3. Compute the number of kilograms of hydrogen that pass per hour through a 5-mm-thick sheet of palladium having an area of 0.20 m2 at 500C. Assume a diffusion coefficient of 1.0 108 m2 /s, that the concentrations at the high- and low-pressure sides of the plate are 2.4 and 0.6 kg of hydrogen per cubic meter of palladium, and that steady-state conditions have been attained

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1
You know the right answer?
The purification of hydrogen gas by diffusion through a palladium sheet was discussed in Section 5.3...
Questions


Biology, 14.05.2020 05:57




Geography, 14.05.2020 05:57


Mathematics, 14.05.2020 05:57


Mathematics, 14.05.2020 05:57

English, 14.05.2020 05:57



Mathematics, 14.05.2020 05:57

History, 14.05.2020 05:57

Mathematics, 14.05.2020 05:57



Mathematics, 14.05.2020 05:57

Advanced Placement (AP), 14.05.2020 05:57

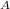 = 2.4
= 2.4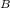 = 0.6 kg
= 0.6 kg

