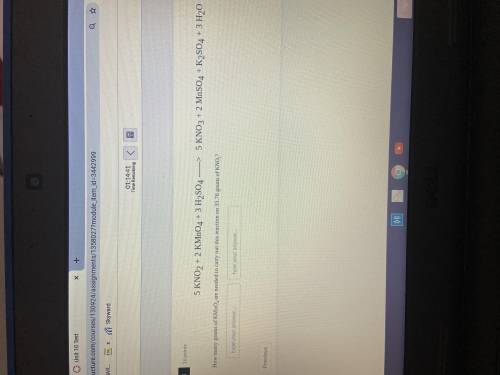
How many grams of KMnO4 are needed to carry out this reaction on 35.76 grams of KNO2
...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
You know the right answer?
Questions

Advanced Placement (AP), 30.08.2021 19:40

World Languages, 30.08.2021 19:40



Mathematics, 30.08.2021 19:40

Mathematics, 30.08.2021 19:40

Mathematics, 30.08.2021 19:40



History, 30.08.2021 19:40




English, 30.08.2021 19:40

Mathematics, 30.08.2021 19:40


Mathematics, 30.08.2021 19:40

English, 30.08.2021 19:40

Mathematics, 30.08.2021 19:40