
Chemistry, 13.03.2021 01:00 mvtthewisdead
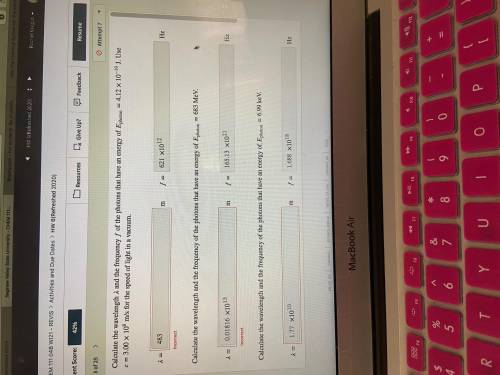
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J. Use =3.00×108 m/s for the speed of light in a vacuum.
Calculate the wavelength and the frequency of the photons that have an energy of photon=683 MeV.
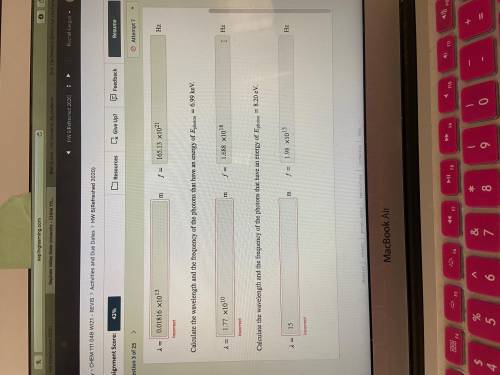
Calculate the wavelength and the frequency of the photons that have an energy of photon=6.99 keV.
Calculate the wavelength and the frequency of the photons that have an energy of photon=8.20 eV.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J...
Questions

Chemistry, 12.10.2020 15:01

Mathematics, 12.10.2020 15:01





Chemistry, 12.10.2020 15:01



History, 12.10.2020 15:01

Computers and Technology, 12.10.2020 15:01

SAT, 12.10.2020 15:01

Computers and Technology, 12.10.2020 15:01

Physics, 12.10.2020 15:01



Mathematics, 12.10.2020 15:01





