Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
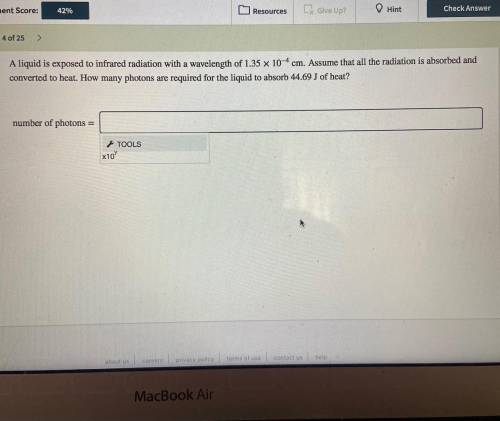
A liquid is exposed to infrared radiation with a wavelength of 1.35×10−4 cm.
Assume that all the ra...
Questions


World Languages, 30.07.2019 05:30

Physics, 30.07.2019 05:30

Social Studies, 30.07.2019 05:30

Computers and Technology, 30.07.2019 05:30



Mathematics, 30.07.2019 05:30




Biology, 30.07.2019 05:30




Computers and Technology, 30.07.2019 05:30



Spanish, 30.07.2019 05:30