
Chemistry, 13.03.2021 04:00 youngcie04
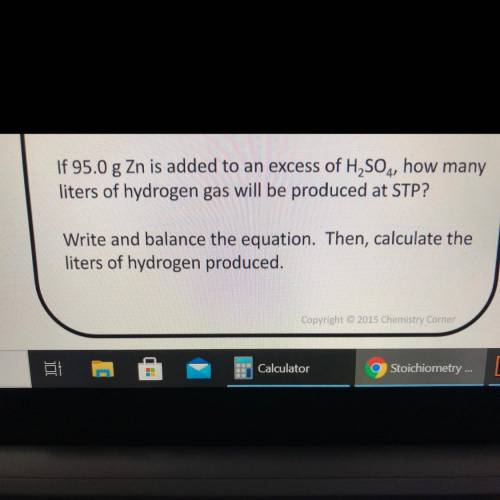
If 95.0 g Zn is added to an excess of H2SO4, how many
liters of hydrogen gas will be produced at STP?
Write and balance the equation. Then, calculate the
liters of hydrogen produced.
Please help

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
If 95.0 g Zn is added to an excess of H2SO4, how many
liters of hydrogen gas will be produced at ST...
Questions


Mathematics, 04.08.2019 01:00

Mathematics, 04.08.2019 01:00


Mathematics, 04.08.2019 01:00



Mathematics, 04.08.2019 01:00




Mathematics, 04.08.2019 01:00



Advanced Placement (AP), 04.08.2019 01:00


Mathematics, 04.08.2019 01:00

Biology, 04.08.2019 01:00


History, 04.08.2019 01:00



