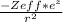Chemistry, 13.03.2021 05:50 HeyItsCookie9605
Describe the critical property ofelectrons in 2s versus 2p orbitals that causes the radial distribution functions of these orbitals to have different shapes about the nucleus such that 2s electrons effectively penetrate closer to the nucleus than do electrons in 2p orbitals. Then, write an expression for the potential energy function describing this effect.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2



Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
Describe the critical property ofelectrons in 2s versus 2p orbitals that causes the radial distribut...
Questions




Geography, 25.05.2021 17:30

English, 25.05.2021 17:30



English, 25.05.2021 17:30


Mathematics, 25.05.2021 17:30




Mathematics, 25.05.2021 17:30

Mathematics, 25.05.2021 17:30

Mathematics, 25.05.2021 17:30