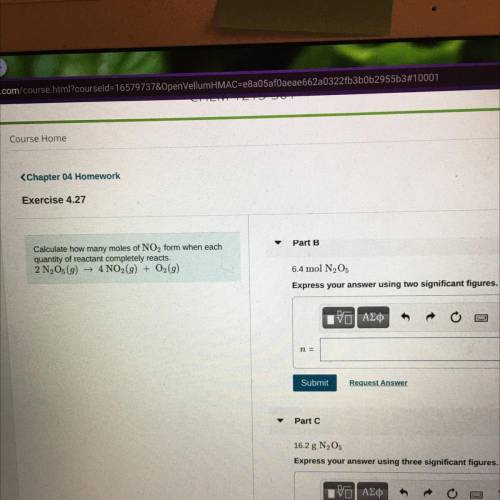
Calculate how many moles of NO2 form when each
quantity of reactant completely reacts.
2 N2O5...

Calculate how many moles of NO2 form when each
quantity of reactant completely reacts.
2 N2O5(9) + 4NO2(g) + O2(9)
Part B
6.4 mol N205
Express your answer using two significant figures.
VALO
n =
Submit
Request Answer
Part C
16.2 g N205
Express your answer using three significant figurer

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
Questions


Mathematics, 25.01.2021 02:20

Mathematics, 25.01.2021 02:20



Mathematics, 25.01.2021 02:30

Mathematics, 25.01.2021 02:30


English, 25.01.2021 02:30

Mathematics, 25.01.2021 02:30


Mathematics, 25.01.2021 02:30

Mathematics, 25.01.2021 02:30





English, 25.01.2021 02:30


Business, 25.01.2021 02:30



