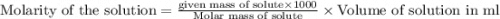Please if you know help
please
the formula of copper sulfate crystals is CuSo4.5H2O. 40...

Chemistry, 13.03.2021 14:00 tiffxnnyyy
Please if you know help
please
the formula of copper sulfate crystals is CuSo4.5H2O. 400ml of 0.02mol/l copper (ii) sulfate solution is needed to be prepered .
a. calculate the molar mass of the solute .
b. calculate the mass of the solute that sould be weighted to prepare this solution .
c. describe in details the steps of preperation of the solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
Questions


History, 05.03.2021 16:20

Mathematics, 05.03.2021 16:20

Mathematics, 05.03.2021 16:20


Mathematics, 05.03.2021 16:20

Social Studies, 05.03.2021 16:20

History, 05.03.2021 16:20



Biology, 05.03.2021 16:30

Mathematics, 05.03.2021 16:30

Social Studies, 05.03.2021 16:30



English, 05.03.2021 16:30

Mathematics, 05.03.2021 16:30



Chemistry, 05.03.2021 16:30

 and dissolve in water until the volume is 400 ml
and dissolve in water until the volume is 400 ml