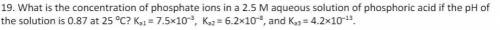Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
What is the concentration of phosphate ions in a 2.5 M aqueous solution of phosphoric acid if the pH...
Questions



Mathematics, 21.12.2020 02:00

Mathematics, 21.12.2020 02:00

Social Studies, 21.12.2020 02:00

Mathematics, 21.12.2020 02:00


Biology, 21.12.2020 02:00


History, 21.12.2020 02:10


Mathematics, 21.12.2020 02:10

Health, 21.12.2020 02:10

English, 21.12.2020 02:10

Mathematics, 21.12.2020 02:10


Mathematics, 21.12.2020 02:10



Health, 21.12.2020 02:10