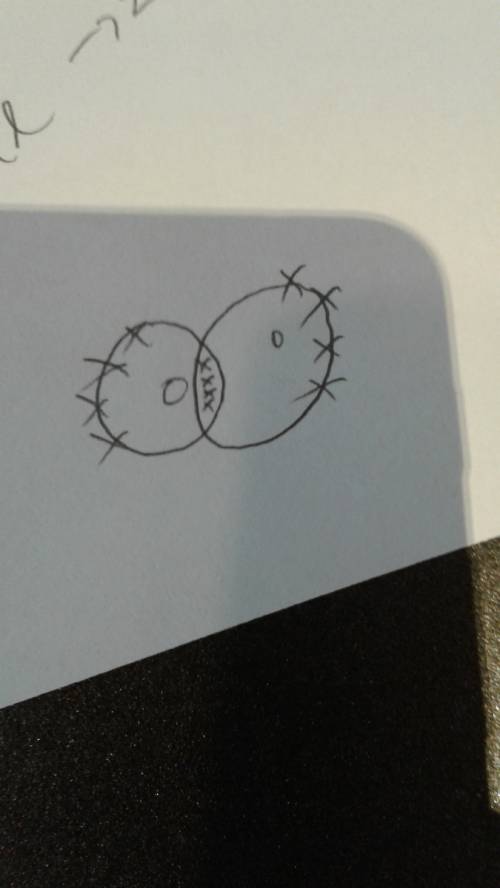
An oxygen atom has six electrons in its outermost energy level.
explain why two oxygen at...

Chemistry, 29.09.2019 18:30 BreBreDoeCCx
An oxygen atom has six electrons in its outermost energy level.
explain why two oxygen atoms must share four electrons when they form a covalent bond?
answer in complete sentences and thoroughly. 5-7

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Questions

Social Studies, 08.01.2020 21:31

English, 08.01.2020 21:31


History, 08.01.2020 21:31


Mathematics, 08.01.2020 21:31

Biology, 08.01.2020 21:31

Geography, 08.01.2020 21:31

Spanish, 08.01.2020 21:31


Mathematics, 08.01.2020 21:31

Mathematics, 08.01.2020 21:31


Chemistry, 08.01.2020 21:31

Biology, 08.01.2020 21:31

Arts, 08.01.2020 21:31


Mathematics, 08.01.2020 21:31


Mathematics, 08.01.2020 21:31