
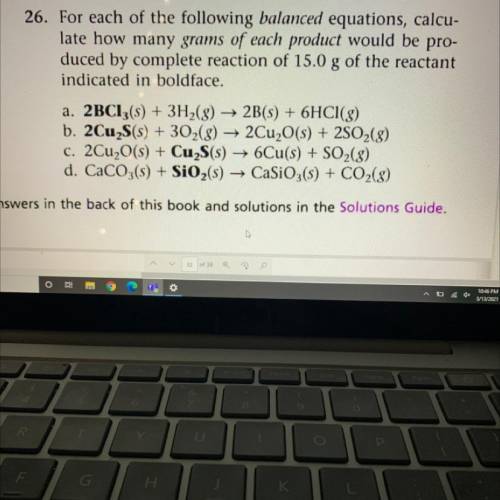
For each of the following balanced equations, calculate how many grams of each product would be produced by complete reaction of 15.0 g of the reactant
indicated in boldface. (*)
a. *2BC13(s)* + 3H2(g) -> 2B(s) + 6HCl(g)
b. *2Cu S(s)* + 302(g) → 2Cu2O(s) + 2SO2(g)
C. 2Cu2O(s) + *Cu2S(s)* -> 6Cu(s) + SO2(g)
d. CaCO3(s) + *SiO2(s)* → CaSiO3(s) + CO2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
For each of the following balanced equations, calculate how many grams of each product would be prod...
Questions

English, 31.03.2021 14:00

Mathematics, 31.03.2021 14:00

Mathematics, 31.03.2021 14:00



Geography, 31.03.2021 14:00

Biology, 31.03.2021 14:00



English, 31.03.2021 14:00

Mathematics, 31.03.2021 14:00

Mathematics, 31.03.2021 14:00


Mathematics, 31.03.2021 14:00

Mathematics, 31.03.2021 14:00



Mathematics, 31.03.2021 14:00


English, 31.03.2021 14:00



