
Chemistry, 17.03.2021 23:50 JamesLachoneus
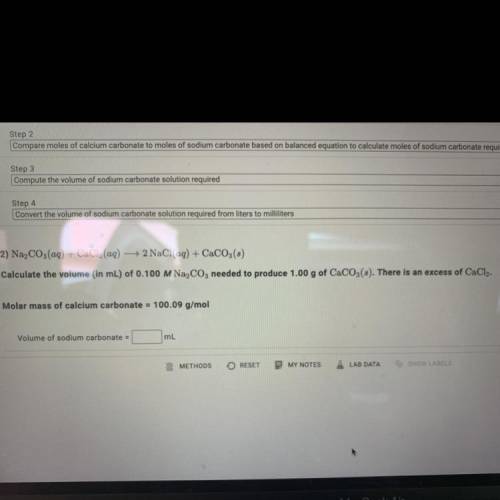
Calculate the volume (in mL) of 0.100 M Na, C03 needed to produce 1.00 g of CaCO3(s).
There is an excess of CaCl2.
Molar mass of calcium carbonate = 100.09 g/mol
Volume of sodium carbonate = ?mL

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3


Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
Calculate the volume (in mL) of 0.100 M Na, C03 needed to produce 1.00 g of CaCO3(s).
There is an e...
Questions


Mathematics, 18.02.2020 01:46



History, 18.02.2020 01:47



Mathematics, 18.02.2020 01:47

Mathematics, 18.02.2020 01:47


English, 18.02.2020 01:47


Computers and Technology, 18.02.2020 01:47









