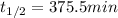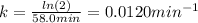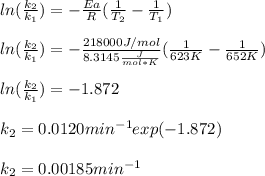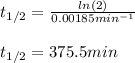Chemistry, 18.03.2021 01:00 21ghostrider21
The decomposition of ethylene oxide (CH₂)₂O(g) → CH₄(g) + CO(g) is a first order reaction with a half-life of 58.0 min at 652 K. The activation energy of the reaction is 218 kJ/mol. Calculate the half-life at 623 K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1


You know the right answer?
The decomposition of ethylene oxide (CH₂)₂O(g) → CH₄(g) + CO(g) is a first order reaction with a hal...
Questions


Chemistry, 03.10.2021 21:10


Mathematics, 03.10.2021 21:10



Mathematics, 03.10.2021 21:10

History, 03.10.2021 21:10

Mathematics, 03.10.2021 21:10







Mathematics, 03.10.2021 21:10

Mathematics, 03.10.2021 21:10



Chemistry, 03.10.2021 21:10