
HELPP PLS WITH GIVE POINTS AND BRAINLIEST‼️‼️‼️‼️
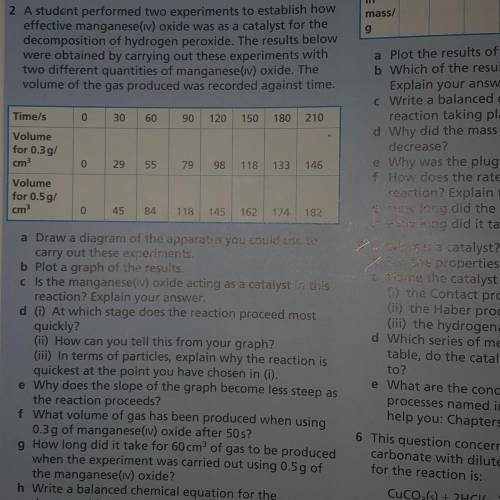
2 A student performed two experiments to establish how
effective manganese(iv) oxide was as a catalyst for the
decomposition of hydrogen peroxide. The results below
were obtained by carrying out these experiments with
two different quantities of manganese(iv) oxide. The
volume of the gas produced was recorded against time.
Time/s
0
30
60
90 120 150 180
210
0
29
55
79
98
118 133
146
Volume
for 0.3g/
cm
Volume
for 0.5g/
cm
0
45
84
118 145 162 174 182
a Draw a diagram of the apparatus you could use to
carry out these experiments.
b Plot a graph of the results.
c Is the manganese(IV) oxide acting as a catalyst in this
reaction? Explain your answer.
d (i) At which stage does the reaction proceed most
quickly?
(ii) How can you tell this from your graph?
(iii) In terms of particles, explain why the reaction is
quickest at the point you have chosen in (i).
e Why does the slope of the graph become less steep as
the reaction proceeds?
f What volume of gas has been produced when using
0.3g of manganese(iv) oxide after 50 s?
g How long did it take for 60 cm of gas to be produced
when the experiment was carried out using 0.5g of
the manganese(iv) oxide?
h Write a balanced chemical equation for the
decomposition of hydrogen peroxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3


Chemistry, 23.06.2019 10:30
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
You know the right answer?
HELPP PLS WITH GIVE POINTS AND BRAINLIEST‼️‼️‼️‼️
2 A student performed two experiments to establis...
Questions





History, 06.05.2020 20:15






English, 06.05.2020 20:15

Mathematics, 06.05.2020 20:15

Mathematics, 06.05.2020 20:15



Mathematics, 06.05.2020 20:16






