
Chemistry, 18.03.2021 01:20 alondra6190
Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless.
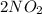 (g) ⇌
(g) ⇌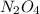 (g)
(g)
When the mixture was moved from room temperature to a higher temperature, the mixture turned darker brown. Which of the following conclusions about the mixture is true?
A. The forward reaction is exothermic.
B. The backward reaction has a negative net enthalpy.
C. The colorless gas has a higher enthalpy than the brown gas.
I know that is an exothermic reaction, but it shifts to left, so it should be a backward reaction, not a forward. Therefore, I don't think it is A. I am leaning towards B, but I would like someone to double-check my thinking. Please no fake answers or external links. Thanks in advance!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1


Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas...
Questions


Chemistry, 20.03.2021 21:10

English, 20.03.2021 21:10


Mathematics, 20.03.2021 21:10


History, 20.03.2021 21:10

Mathematics, 20.03.2021 21:10

Mathematics, 20.03.2021 21:10

Mathematics, 20.03.2021 21:10

Mathematics, 20.03.2021 21:10



Mathematics, 20.03.2021 21:10


Mathematics, 20.03.2021 21:10

English, 20.03.2021 21:10


Chemistry, 20.03.2021 21:10



