
Chemistry, 18.03.2021 01:40 baileyanne9389
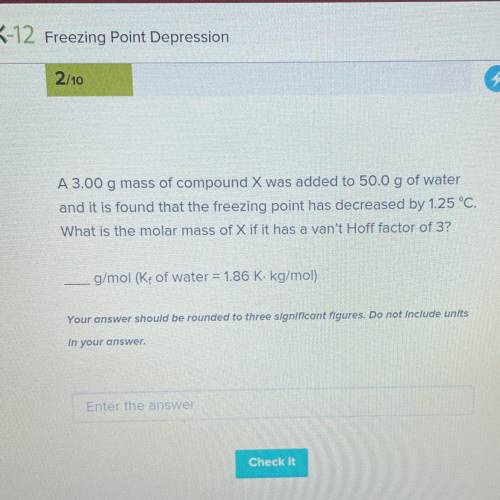
A 3.00 g mass of compound X was added to 50.0 g of water
and it is found that the freezing point has decreased by 1.25 °C.
What is the molar mass of X if it has a van't Hoff factor of 3?
g/mol (K, of water = 1.86 K. kg/mol)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
A 3.00 g mass of compound X was added to 50.0 g of water
and it is found that the freezing point ha...
Questions





Mathematics, 10.10.2020 17:01

Social Studies, 10.10.2020 17:01

Mathematics, 10.10.2020 17:01


Mathematics, 10.10.2020 17:01






Mathematics, 10.10.2020 17:01


Mathematics, 10.10.2020 17:01

English, 10.10.2020 17:01




