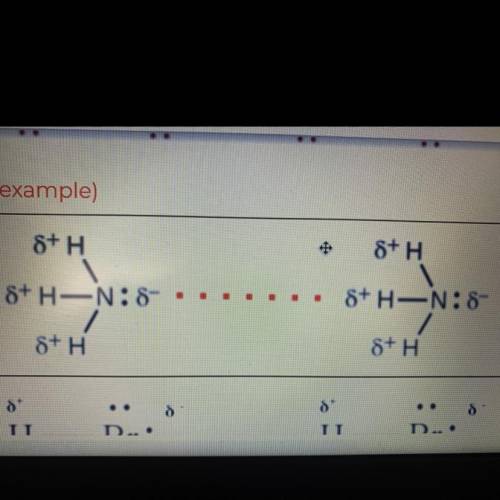
Hydrogen bonding, dipole-dipole, or dispersion forces
And why?
...

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

You know the right answer?
Questions

Mathematics, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57

Health, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57

Chemistry, 16.06.2020 05:57



Mathematics, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57

Chemistry, 16.06.2020 05:57




Mathematics, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57