1. What is the wavelength (in meters) of a radio wave with a frequency of 9.31 x Hz?
2....

Chemistry, 18.03.2021 02:00 nathaniel12
1. What is the wavelength (in meters) of a radio wave with a frequency of 9.31 x 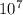 Hz?
Hz?
2. Calculate the energy of:
a) a photon with a wavelength of 5.00 x 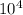 nm.
nm.
b) a photon with a wavelength of 5.00 x 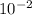 nm.
nm.
3. The energy of a photon is 5.87 x 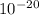 J. What is its wavelength in nm?
J. What is its wavelength in nm?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1


You know the right answer?
Questions

Mathematics, 16.12.2020 17:10

English, 16.12.2020 17:10



Mathematics, 16.12.2020 17:10

Computers and Technology, 16.12.2020 17:10

Mathematics, 16.12.2020 17:10

Mathematics, 16.12.2020 17:10

Mathematics, 16.12.2020 17:10

Biology, 16.12.2020 17:10

Arts, 16.12.2020 17:10



English, 16.12.2020 17:10



Mathematics, 16.12.2020 17:10


Biology, 16.12.2020 17:10



