
Chemistry, 18.03.2021 02:10 chamarabrown9260
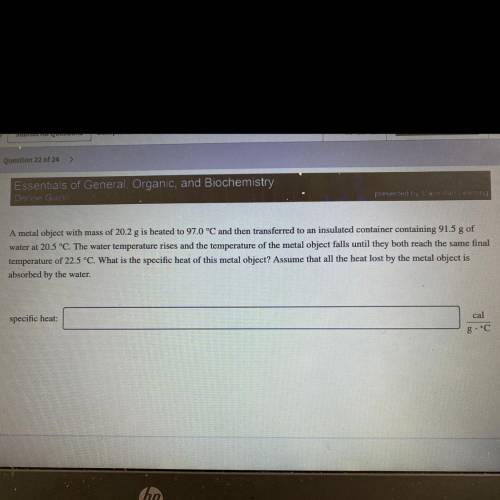
A metal object with mass of 20.2 g is heated to 97.0 °C and then transferred to an insulated container containing 91.5 g of
water at 20.5 °C. The water temperature rises and the temperature of the metal object falls until they both reach the same final
temperature of 22.5 °C. What is the specific heat of this metal object? Assume that all the heat lost by the metal object is
absorbed by the water.
specific heat: cal/gal •°C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2


Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
A metal object with mass of 20.2 g is heated to 97.0 °C and then transferred to an insulated contain...
Questions

English, 26.05.2020 19:01

English, 26.05.2020 19:01

Computers and Technology, 26.05.2020 19:01

Mathematics, 26.05.2020 19:01

Chemistry, 26.05.2020 19:01




Social Studies, 26.05.2020 19:01

Mathematics, 26.05.2020 19:01

History, 26.05.2020 19:01



Social Studies, 26.05.2020 19:01

Arts, 26.05.2020 19:01


Mathematics, 26.05.2020 19:01


History, 26.05.2020 19:01

Mathematics, 26.05.2020 19:01



