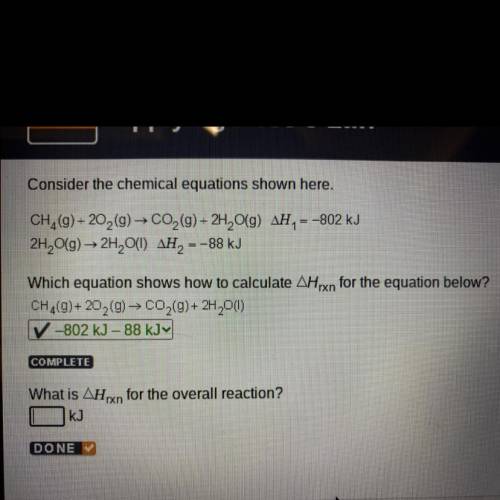
Consider the chemical equations shown here.
CH (9) +20,(9) → CO2 (9)+ 2H, O(9) AH, = -802 kJ
...

Chemistry, 18.03.2021 02:40 graceduke2005p6z8yp
Consider the chemical equations shown here.
CH (9) +20,(9) → CO2 (9)+ 2H, O(9) AH, = -802 kJ
2H2O(g) → 2H, O(1) AH, -
= -88 kJ
Which equation shows how to calculate Arxn for the equation below?
CH,(9) + 20 (0) - CO,(9)+ 2H 0(1)
-802 kJ - 88 KJY
COMPLETE
What is AHxn for the overall reaction?
KJ
PLEASE FOCUS ON THE SECOND PART !!
Help

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
Questions

Health, 13.04.2021 14:00





Computers and Technology, 13.04.2021 14:00

Arts, 13.04.2021 14:00


English, 13.04.2021 14:00


English, 13.04.2021 14:00


Mathematics, 13.04.2021 14:00

Mathematics, 13.04.2021 14:00



Mathematics, 13.04.2021 14:00

Mathematics, 13.04.2021 14:00




