
Chemistry, 18.03.2021 02:50 Fireburntbudder
A gas at 1.10 atm and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the temperature is raised to 80.0°C and the pressure increased to 2.40 atm, what is the new volume?
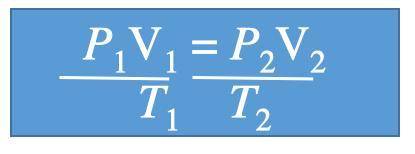

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1


Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
A gas at 1.10 atm and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the tem...
Questions

Arts, 16.11.2020 01:00



Mathematics, 16.11.2020 01:00

Mathematics, 16.11.2020 01:00



Mathematics, 16.11.2020 01:00



Business, 16.11.2020 01:00




Physics, 16.11.2020 01:00


Arts, 16.11.2020 01:00

Mathematics, 16.11.2020 01:00

English, 16.11.2020 01:00

Mathematics, 16.11.2020 01:00



