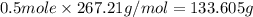Chemistry, 21.09.2019 23:30 angie07253
Given the following equation, how many grams of pbco3 will dissolve when exactly 1.0 l of 1.00 m h+ is added to 6.00 g of pbco3?
when the equation is pbco3(s) + 2h(+)(aq) = pb(2+)(aq) + h2o(l) + co2(g)
explain in detail, step by step

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
Given the following equation, how many grams of pbco3 will dissolve when exactly 1.0 l of 1.00 m h+...
Questions



History, 10.11.2021 21:30


Mathematics, 10.11.2021 21:30

Mathematics, 10.11.2021 21:30

Business, 10.11.2021 21:30

English, 10.11.2021 21:30

History, 10.11.2021 21:30





Law, 10.11.2021 21:30

Mathematics, 10.11.2021 21:30


Mathematics, 10.11.2021 21:30

Mathematics, 10.11.2021 21:30


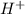 solution.
solution.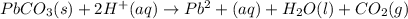
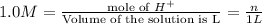
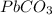 then , 1 mole will dissolve ;
then , 1 mole will dissolve ;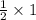 moles of
moles of