
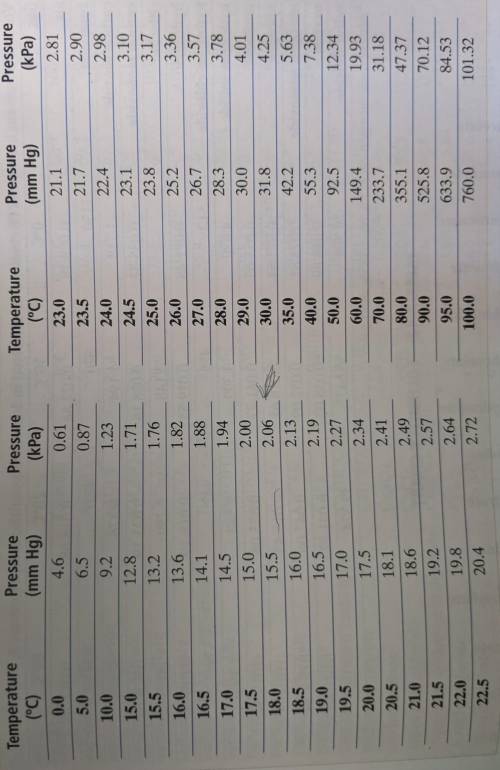
Can someone please help? This was due yesterday. The Appendix Table A-8 is linked in a photo. A certain mass of oxygen was collected over water when potassium chlorate was decomposed by heating. The volume of the oxygen sample collected was 720. mL at 25.0° C and a barometric pressure of 755 torr. What would the volume of the oxygen be at STP? (Hint: First calculate the partial pressure of the oxygen, using Appendix Table A-8. Then use the combined gas law.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

You know the right answer?
Can someone please help? This was due yesterday. The Appendix Table A-8 is linked in a photo.
A cer...
Questions




Biology, 22.10.2020 16:01

Chemistry, 22.10.2020 16:01


Computers and Technology, 22.10.2020 16:01

English, 22.10.2020 16:01





Computers and Technology, 22.10.2020 16:01

Chemistry, 22.10.2020 16:01

Mathematics, 22.10.2020 16:01

Mathematics, 22.10.2020 16:01

English, 22.10.2020 16:01


Mathematics, 22.10.2020 16:01



