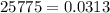Chemistry, 18.03.2021 03:10 hiiamsuperverylong
Methane gas is collected over water at 25 C and an atmospheric pressure of 775 mmHg. What is the dry pressure of the gas at this temperature? (Vapor Pressure of water = 0.0313 atm).

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
Methane gas is collected over water at 25 C and an atmospheric pressure of 775 mmHg. What is the dry...
Questions


Mathematics, 20.06.2020 05:57

Mathematics, 20.06.2020 05:57

English, 20.06.2020 05:57


History, 20.06.2020 05:57

History, 20.06.2020 05:57






Geography, 20.06.2020 05:57

Mathematics, 20.06.2020 05:57





Mathematics, 20.06.2020 05:57

Mathematics, 20.06.2020 05:57