
Chemistry, 18.03.2021 03:20 LarryJoeseph
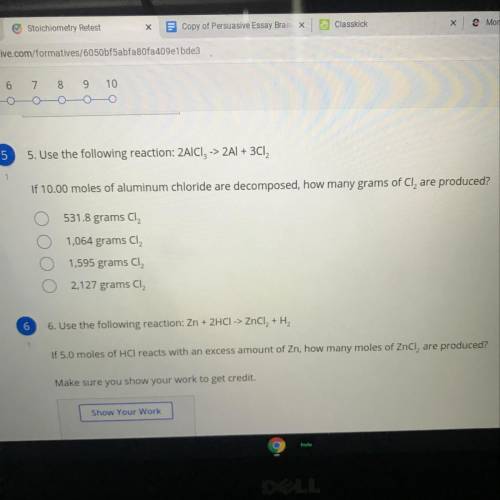
Use the following reaction: 2AICI, -> 2Al + 3Cl2
If 10.00 moles of aluminum chloride are decomposed, how many grams of Cl, are produced?
A. 531.8 grams Cl2
B. 1,064 grams CI2
C. 1,595 grams Cl2
D. 2,127 grams CI2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Use the following reaction: 2AICI, -> 2Al + 3Cl2
If 10.00 moles of aluminum chloride are decompo...
Questions


Medicine, 06.02.2022 03:20

Social Studies, 06.02.2022 03:20

English, 06.02.2022 03:20

English, 06.02.2022 03:20

Mathematics, 06.02.2022 03:20

World Languages, 06.02.2022 03:20

Social Studies, 06.02.2022 03:20


English, 06.02.2022 03:20

Mathematics, 06.02.2022 03:20

Social Studies, 06.02.2022 03:20


Business, 06.02.2022 03:20

World Languages, 06.02.2022 03:20

English, 06.02.2022 03:30

Mathematics, 06.02.2022 03:30


Mathematics, 06.02.2022 03:30



