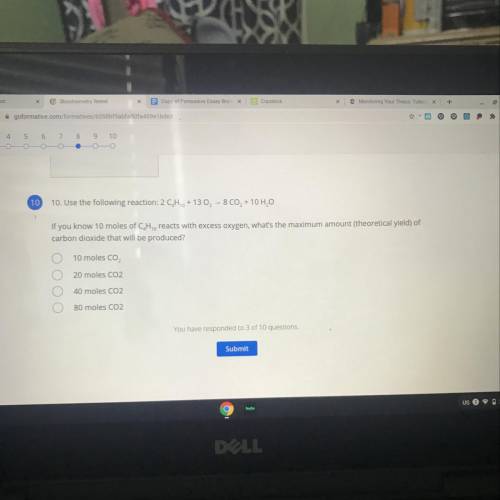
Use the following reaction:
2 CH2 + 13 O2 - 8 CO2 + 10 H2O
If you know 10 moles of C, H, reac...

Chemistry, 18.03.2021 03:30 anonymousanon
Use the following reaction:
2 CH2 + 13 O2 - 8 CO2 + 10 H2O
If you know 10 moles of C, H, reacts with excess oxygen, what's the maximum amount (theoretical yield) of
carbon dioxide that will be produced?
A. 10 moles CO,
B. 20 moles CO2
C. 40 moles CO2
D. 80 moles CO2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
Questions

Mathematics, 22.11.2019 08:31



Social Studies, 22.11.2019 08:31






Mathematics, 22.11.2019 08:31

Health, 22.11.2019 08:31


Chemistry, 22.11.2019 08:31

History, 22.11.2019 08:31



Mathematics, 22.11.2019 08:31

Mathematics, 22.11.2019 08:31


Biology, 22.11.2019 08:31



