
Chemistry, 18.03.2021 03:30 querline87
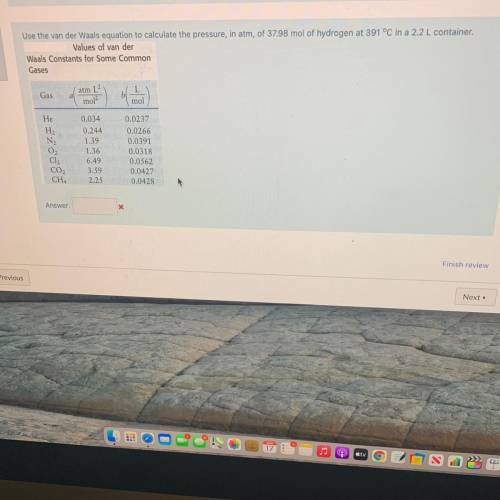
Use the van der Waals equation to calculate the pressure, in atm of 37.98 mol of hydrogen at 391 °C in a 2.2 L container.
Values of van der
Waals Constants for Some Common
Gases
Gas
atm 1
mol
mol
He
Н.
0.034
0.244
1.39
1.36
0.0237
0.0266
0.0391
0.0318
0.0562
0.0427
0.0428
3.59
2.25
X

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Use the van der Waals equation to calculate the pressure, in atm of 37.98 mol of hydrogen at 391 °C...
Questions

Business, 18.11.2019 11:31

English, 18.11.2019 11:31

Mathematics, 18.11.2019 11:31



Mathematics, 18.11.2019 11:31

Mathematics, 18.11.2019 11:31

English, 18.11.2019 11:31



English, 18.11.2019 11:31


History, 18.11.2019 11:31

History, 18.11.2019 11:31






Physics, 18.11.2019 11:31



