How does Nitrogen Dioxide
react with :
I. Copper
ii. Phosphorus.
iii. Sodium h...

Chemistry, 18.03.2021 04:40 BreBreDoeCCx
How does Nitrogen Dioxide
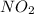
react with :
I. Copper
ii. Phosphorus.
iii. Sodium hydroxide.
Note you are asked to explain the reaction that occurs between Nitrogen Dioxide and each of the given elements with the aid of a chemical equation.
State what type of reaction that occurs.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Questions

Mathematics, 16.11.2020 21:40



Mathematics, 16.11.2020 21:40

Mathematics, 16.11.2020 21:40






Mathematics, 16.11.2020 21:40




English, 16.11.2020 21:40


Biology, 16.11.2020 21:40


English, 16.11.2020 21:40



