
Chemistry, 18.03.2021 07:20 makaylagrandsta
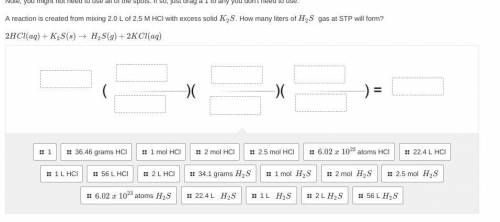
Please only answer w help and i will give you brainliest. <3 A reaction is created from mixing 2.0 L of 2.5 M HCl with excess solid K2S. How many liters of H2S gas at STP will form?
2HCl(aq)+K2S(s)→ H2S(g)+2KCl(aq)
(picture below)
-
C3H8+5O2 → 3 CO2 +4H2O
If 33.6 liters of O2 gas at STP react with 55 grams of C3H8, what is the limiting reactant? How much water can be formed (in grams)?
thank you!!!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3


Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

You know the right answer?
Please only answer w help and i will give you brainliest. <3 A reaction is created from mixing 2....
Questions


Biology, 30.01.2020 06:45

Biology, 30.01.2020 06:45


History, 30.01.2020 06:46


History, 30.01.2020 06:46


Mathematics, 30.01.2020 06:46

Social Studies, 30.01.2020 06:46




Biology, 30.01.2020 06:46

Mathematics, 30.01.2020 06:46








