How many moles of CO2 are produced
when 10.0 moles of C3H8 are burned in
excess oxygen?
...

Chemistry, 18.03.2021 08:50 mrylenastewart
How many moles of CO2 are produced
when 10.0 moles of C3H8 are burned in
excess oxygen?
C3H8 + 5 O2 ---> 3 CO2 + 4H2O

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
Questions


Mathematics, 18.04.2021 05:10


History, 18.04.2021 05:10



Mathematics, 18.04.2021 05:10



Geography, 18.04.2021 05:10


Mathematics, 18.04.2021 05:10

English, 18.04.2021 05:10







Mathematics, 18.04.2021 05:10

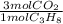 = 30.0 mol CO₂
= 30.0 mol CO₂

