
Chemistry, 18.03.2021 09:10 xelynncaldera
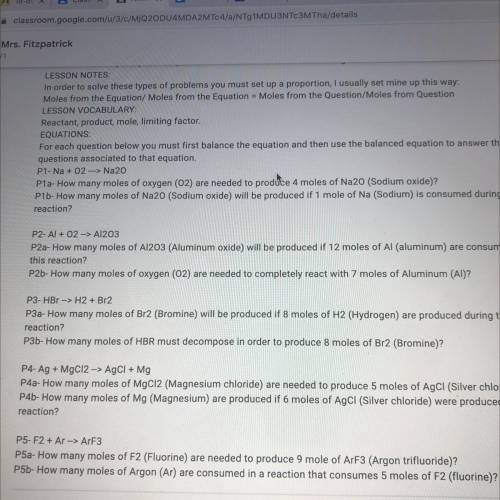
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (Argon trifluoride)?
P5b- How many moles of Argon (Ar) are consumed in a reaction that consumes 5 moles of F2 (fluorine)?
I need the answers for the p5 part please help

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (A...
Questions


History, 22.01.2021 01:10


Mathematics, 22.01.2021 01:10


Computers and Technology, 22.01.2021 01:10


Arts, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10




History, 22.01.2021 01:10

Computers and Technology, 22.01.2021 01:10

Social Studies, 22.01.2021 01:10



Arts, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10



