
Chemistry, 18.03.2021 09:00 jazzy76783
6HCl(aq) + 2A1)
-
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1.20 moles of aluminum?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2
You know the right answer?
6HCl(aq) + 2A1)
-
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1...
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1...
Questions

Computers and Technology, 20.03.2020 07:02




Computers and Technology, 20.03.2020 07:03

Computers and Technology, 20.03.2020 07:03

English, 20.03.2020 07:03


Mathematics, 20.03.2020 07:03

Mathematics, 20.03.2020 07:03


English, 20.03.2020 07:03


Mathematics, 20.03.2020 07:04





History, 20.03.2020 07:04

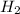 will be produced 1.20 moles of aluminium.
will be produced 1.20 moles of aluminium.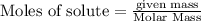
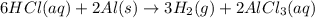
 produce = 3 moles of
produce = 3 moles of 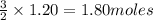 of
of 

