
Chemistry, 19.09.2019 12:10 aaminohasan142
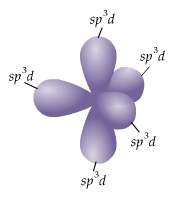
Which of the following clusters of orbitals could form a shape similar to that shown here (figure 3) in the valence shell of an isolated atom or one about to enter into bonding with other atoms?
a) five sp³d
b) three sp² and one p orbital

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

You know the right answer?
Which of the following clusters of orbitals could form a shape similar to that shown here (figure 3)...
Questions


Computers and Technology, 09.03.2021 21:50



Mathematics, 09.03.2021 21:50

Computers and Technology, 09.03.2021 21:50

Mathematics, 09.03.2021 21:50

Mathematics, 09.03.2021 21:50

Mathematics, 09.03.2021 21:50





Biology, 09.03.2021 21:50




History, 09.03.2021 21:50


History, 09.03.2021 21:50



