When two volatile liquids (x and y) are mixed, the solution process involves
1. breaking the...

When two volatile liquids (x and y) are mixed, the solution process involves
1. breaking the intermolecular and attractions, and
2. forming new attractions.
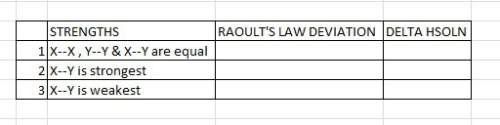
complete this table describing how the relative strengths of these attractive forces affect vapor pressure and enthalpy of solution. (see attachment)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
Questions








Mathematics, 29.07.2019 19:00

Health, 29.07.2019 19:00


Biology, 29.07.2019 19:00



Biology, 29.07.2019 19:00

Mathematics, 29.07.2019 19:00


History, 29.07.2019 19:00

Mathematics, 29.07.2019 19:00

History, 29.07.2019 19:00

Advanced Placement (AP), 29.07.2019 19:00



