
Chemistry, 18.03.2021 20:50 aroman4511
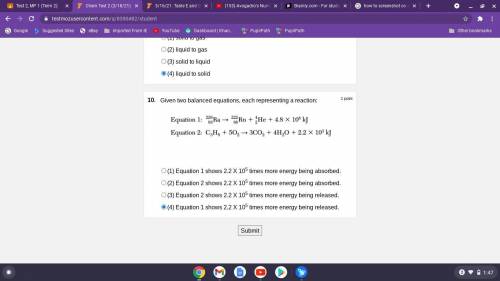
Given two balanced equations, each representing a reaction:
(1) Equation 1 shows 2.2 X 105 times more energy being absorbed. (\
2) Equation 2 shows 2.2 X 105 times more energy being absorbed.
(3) Equation 2 shows 2.2 X 105 times more energy being released.
(4) Equation 1 shows 2.2 X 105 times more energy being released.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1

Chemistry, 23.06.2019 14:00
Which of the following represents the balanced reduction half-reaction from the redox reaction? (2 points) pb + pd(no3)2 yields pb(no3)2 + pd pb yields pd2+ + e- pd2+ + 2e- yields pd pb + e- yields pb 2 pd2+ + 4 e- yields 2 pd
Answers: 1
You know the right answer?
Given two balanced equations, each representing a reaction:
(1) Equation 1 shows 2.2 X 105 times mo...
Questions



Mathematics, 18.03.2021 02:30

Social Studies, 18.03.2021 02:30

English, 18.03.2021 02:30

Geography, 18.03.2021 02:30


English, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30



Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

Biology, 18.03.2021 02:30



