
Chemistry, 18.03.2021 21:20 vanitycarraway2000
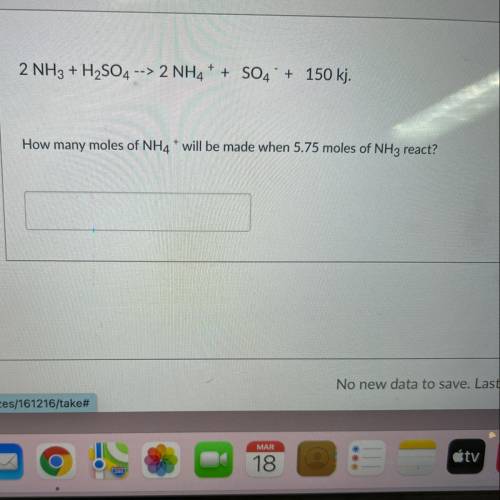
2 NH3 + H2SO4 --> 2NH4+ + SO4 + 150 kj. How many moles of NH4* will be made when 5.75 moles of NH3 react?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1


You know the right answer?
2 NH3 + H2SO4 --> 2NH4+ + SO4 + 150 kj.
How many moles of NH4* will be made when 5.75 moles of N...
Questions

Mathematics, 16.10.2020 07:01




English, 16.10.2020 07:01








English, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01






