Chemistry, 19.03.2021 05:50 taufajane3887
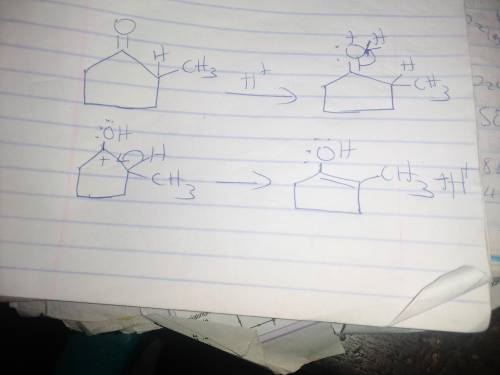
When optically active (S)-2-methylcyclopentanone is treated with an acid (H3O ), the compound loses its optical activity. Explain this observation and draw a mechanism that shows how racemization occurs. For the mechanism, draw the curved arrows as needed. Include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly in your products. Do not use abbreviations such as Me or Ph.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

You know the right answer?
When optically active (S)-2-methylcyclopentanone is treated with an acid (H3O ), the compound loses...
Questions




Social Studies, 23.07.2019 08:30


Mathematics, 23.07.2019 08:30