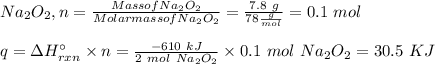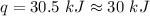Two trials are run, using excess water. In the first trial, 7.8 g of Na2O2(s) (molar mass 78 g/mol) is mixed with 3.2 g of S(s). In the second trial, 7.8 g of Na2O2(s) is mixed with 6.4 g of S(s). The Na2O2(s) and S(s) react as completely as possible. Both trials yield the same amount of SO2(aq). Which of the following identifies the limiting reactant and the heat released, q, for the two trials at 298 K?
Limiting Reactant q
A. S 30. kJ
B. S 61 kJ
C. Na2O2 30. kJ
D. Na2S2 61 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
Two trials are run, using excess water. In the first trial, 7.8 g of Na2O2(s) (molar mass 78 g/mol)...
Questions

Mathematics, 30.04.2021 21:30

Health, 30.04.2021 21:30


Mathematics, 30.04.2021 21:30



Geography, 30.04.2021 21:30


Mathematics, 30.04.2021 21:30


Mathematics, 30.04.2021 21:30

Mathematics, 30.04.2021 21:30

Mathematics, 30.04.2021 21:30

Mathematics, 30.04.2021 21:30


Mathematics, 30.04.2021 21:30




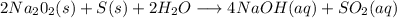
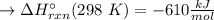
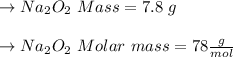
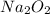 Has been the reactant which is limited since the two experiments are equal to
Has been the reactant which is limited since the two experiments are equal to