
Acetic acid and water react to form hydronium cation and acetate anion, like this:
HCH3CO2(aq) + H2O(l) → H3O^+ (aq) + CH3CO2^-(aq)
Imagine 226. mmol of CH3CO2- are added to a flask containing a mixture of HCH3CO2, H2O, H3O and CH3CO2- at equilibrium, and then answer the following question.
What is the rate of the reverse reaction before any CH3CO2^- has been added to the flask?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1


You know the right answer?
Acetic acid and water react to form hydronium cation and acetate anion, like this:
HCH3CO2(aq) + H2...
Questions

Mathematics, 26.06.2020 18:01

Chemistry, 26.06.2020 18:01





Mathematics, 26.06.2020 18:01




Mathematics, 26.06.2020 18:01









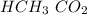 , to which 3 is added. In balance, that rate of the forward reaction was its rate with forwarding reaction, both of which are higher than 0 as the response has achieved balance so that both species get a level greater than 0.
, to which 3 is added. In balance, that rate of the forward reaction was its rate with forwarding reaction, both of which are higher than 0 as the response has achieved balance so that both species get a level greater than 0.

