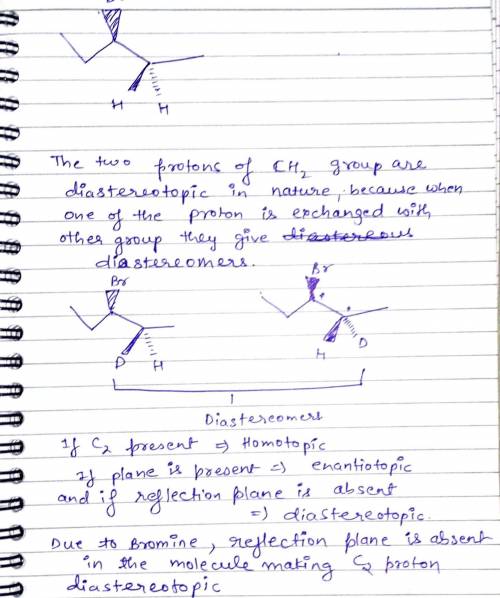
A general rule that the two protons of a CH2 group will be chemically equivalent if there are no chirality centers in the compound. An example of an exception is 3-bromopentane. This compound does not possess a chirality center. Nevertheless, the two highlighted protons are not chemically equivalent. Write down its explaination.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
A general rule that the two protons of a CH2 group will be chemically equivalent if there are no chi...
Questions

English, 10.05.2021 19:30

English, 10.05.2021 19:30



Chemistry, 10.05.2021 19:30

Chemistry, 10.05.2021 19:30


History, 10.05.2021 19:30

Mathematics, 10.05.2021 19:30


Mathematics, 10.05.2021 19:40

Mathematics, 10.05.2021 19:40

Social Studies, 10.05.2021 19:40


Mathematics, 10.05.2021 19:40




Mathematics, 10.05.2021 19:40

Social Studies, 10.05.2021 19:40