
Chemistry, 19.03.2021 20:30 woodfordmaliky
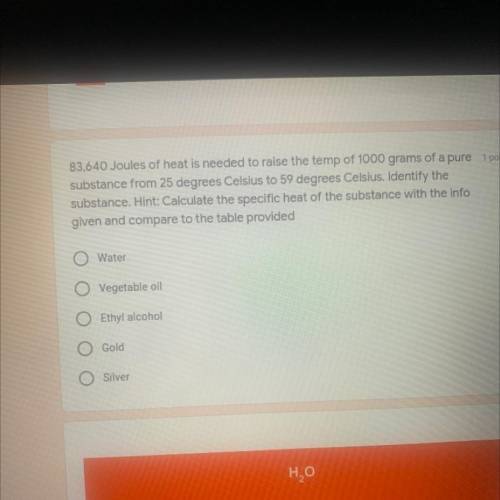
83, 640Joules of heat is needed to raise the temp of 1000 grams of a pure substance from 25 degrees Celsius to 59 degrees Celsius. Identify the substanceHint: Calculate the specific heat of the substance with the info given and compare to the table provided

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1

You know the right answer?
83, 640Joules of heat is needed to raise the temp of 1000 grams of a pure substance from 25 degrees...
Questions






Mathematics, 26.02.2021 20:40


Mathematics, 26.02.2021 20:40


Mathematics, 26.02.2021 20:40



Mathematics, 26.02.2021 20:40

Social Studies, 26.02.2021 20:40

Mathematics, 26.02.2021 20:40





Chemistry, 26.02.2021 20:40



