
Chemistry, 19.03.2021 21:40 lilquongohard
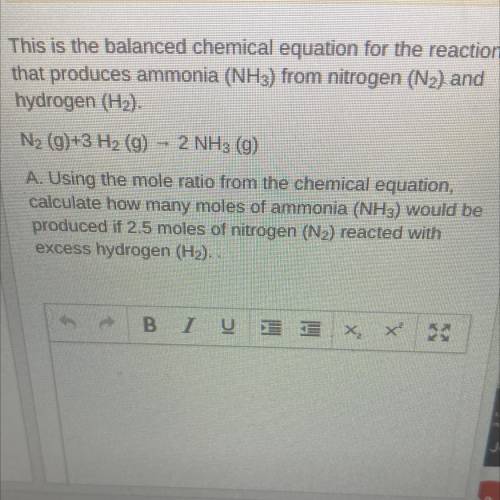
This is the balanced chemical equation for the reaction that produces ammonia (NH3) from nitrogen (N2) and hydrogen (H2).
N2 (g)+3 H2 (g) - 2 NH3 (g)
A. Using the mole ratio from the chemical equation, calculate how many moles of ammonia (NH3) would be produced if 2.5 moles of nitrogen (N2) reacted with
excess hydrogen (H2). .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1
You know the right answer?
This is the balanced chemical equation for the reaction that produces ammonia (NH3) from nitrogen (N...
Questions




Mathematics, 15.12.2020 14:00


Mathematics, 15.12.2020 14:00

Mathematics, 15.12.2020 14:00


Mathematics, 15.12.2020 14:00

Physics, 15.12.2020 14:00

Biology, 15.12.2020 14:00


Mathematics, 15.12.2020 14:00

Chemistry, 15.12.2020 14:00

Mathematics, 15.12.2020 14:00


Mathematics, 15.12.2020 14:00


Social Studies, 15.12.2020 14:00

Mathematics, 15.12.2020 14:00



