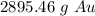Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 21:30
If 1.oo mol cs2 reacts with 1.00 mol o2 , identify the limiting reactant
Answers: 1

Chemistry, 23.06.2019 22:30
Bernard is an excellent chef. however, his beef steak did not turn out well. bernard forgot that steak is tender and has less collagen, and he used the wrong cooking technique. which is the correct technique that bernard should have used? this subject is actually home ec they didnt have it when i had to pick so i chose anyone .
Answers: 1
You know the right answer?
1) 14.7 moles of Au react, how many grams of Au will that be?...
Questions








Chemistry, 25.02.2022 04:50

Mathematics, 25.02.2022 04:50



Computers and Technology, 25.02.2022 04:50

Social Studies, 25.02.2022 04:50

Mathematics, 25.02.2022 04:50

SAT, 25.02.2022 04:50

English, 25.02.2022 04:50



Mathematics, 25.02.2022 04:50

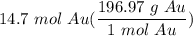 [DA] Multiply [Cancel out units]:
[DA] Multiply [Cancel out units]: