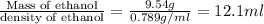Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 05:40
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2

Chemistry, 23.06.2019 11:30
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 300j of energy to the room?unit:
Answers: 1
You know the right answer?
The density of ethanol, C2H5OH, is 0.789 g/mL. How many milliliters of ethanol are needed to produce...
Questions

Mathematics, 14.01.2020 08:31


Mathematics, 14.01.2020 08:31


Mathematics, 14.01.2020 08:31


Social Studies, 14.01.2020 08:31

Health, 14.01.2020 08:31



Chemistry, 14.01.2020 08:31







History, 14.01.2020 08:31

Geography, 14.01.2020 08:31

Health, 14.01.2020 08:31

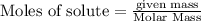
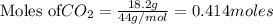
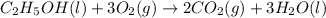
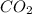 is produced by = 1 mole of
is produced by = 1 mole of 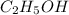
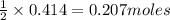 of
of